|
The terminology “blister” as it pertains to spa surfaces actually originated in the marine industry. This phenomenon was experienced in surfaces of boats constructed with gel coat reinforced with glass fibers in polyester resin. In order to understand blisters, it helps to understand a little about polyester resin chemistry. Spa and bath laminating resins are polyester or vinyl ester resins that are formulated for use in ambient temperature, open mold, spray up or hand layup processes. Laminating resins can be formulated for use in the “neat” form or filled with inorganic fillers. They are formulated from several components including polymer, reactive monomer, thixotropic agents, promoters, inhibitors and specialty additives. The specific components and amounts used are dictated by the end-user’s processing requirements, requirements for finished part performance, and cost considerations. Environmental regulations on emissions also effect the resin formulation. The formation of blisters is a complex subject, and they are formed by multiple mechanisms. Most U.S. manufacturers of spas and baths have learned how to eliminate or minimize the causes of blisters, so their incidence has greatly decreased over time. Cook Composite and Polymers Co. (CCP), a leading manufacturer of gel coat and other polyester resins, investigated blister formation in gel coat/FRP applications, and made the following conclusions. There are two basic types of blisters, 1) air or gas in the laminate that expands when heated, and 2) entrapped liquids that expand when heated. There are many sources for the gas and liquids. CCP found that osmotic blisters were common in gel coat/FRP products. Poor application of the laminating resin was another source of air or liquid contamination.
Water, under the right conditions, can reverse this reaction, which is called hydrolysis. Hydrolysis literally means reaction with water. It is a chemical process in which a molecule is cleaved into two parts by the addition of a molecule of water. One fragment of the parent molecule gains a hydrogen ion (H + ) from the additional water molecule. The other group collects the remaining hydroxyl group (OH − ). The most common hydrolysis occurs when a salt of a weak acid or weak base (or both) is dissolved in water. Water autoionizes into negative hydroxyl ions and hydrogen ions. The salt breaks down into positive and negative ions. For example, sodium acetate dissociates in water into sodium and acetate ions. Sodium ions react very little with hydroxyl ions whereas acetate ions combine with hydrogen ions to produce neutral acetic acid, and the net result is a relative excess of hydroxyl ions, causing a basic solution. However, under normal conditions, only a few reactions between water and organic compounds occur. Generally, strong acids or bases must be added in order to achieve hydrolysis where water has no effect. The acid or base is considered a catalyst. They are meant to speed up the reaction, but are recovered at the end of it. Acid–base-catalyzed hydrolyses are very common; one example is the hydrolysis of amides or esters. Their hydrolysis occurs when the nucleophile (a nucleus-seeking agent, e.g., water or hydroxyl ion) attacks the carbon of the carbonyl group of the ester or amide. In an aqueous base, hydroxyl ions are better nucleophiles than dipoles such as water. In acid, the carbonyl group becomes protonated, and this leads to a much easier nucleophilic attack. The products for both hydrolyses are compounds with carboxylic acid groups. Although scientific tests have not been performed in spa conditions, it would follow that water chemistry (acid or base) might effect the rate of hydrolysis. The hydrolysis of the laminating resin, (i.e., the depolymerization of the unsaturated polyester polymer), generates decomposing molecules. Those molecules are dissolved in the water, saturating the laminate and the osmotic pressure cell created. Indeed, on one side of the gel coat (i.e. the membrane) there is water, and on the other side there is a blend of water and decomposition products that are too large to pass through the gel coat. When the pressure created becomes higher than the critical stresses the materials can withstand, (e.g., when the water attack of the laminating resin has been extensive and the concentration of decomposition products has become very high), the structure weakens and blisters are formed. The first portable spas were produced with gelcoat/FRP just like boats. In fact, boat manufacturers were some of the first manufacturers of spas. These early products exhibited the same blistering problem as boats. The performance problems associated with gel coat/FRP in spa service resulted in the development of an improved spa surface material. Since about 1990 most portable or plastic in-ground spas have been produced using a continuous cast, cross-linked acrylic sheet, which is thermoformed to the desired shape. It is then reinforced with polyester reinforced with glass fibers or a special polyurethane coating. However, the industry soon found that blisters were occurring in acrylic spas. Although there has been limited scientific investigation, it is generally accepted that similar processes that occur in gel coat/FRP products also are likely occurring in acrylic spas. It is strongly believed that water is the primary culprit causing blisters. Since bathtubs are not constantly filled with water like a spa, it is rare to have blisters in bathtubs. When they do occur in bathtubs, it is normally in a situation that has had longer water exposure. There are several potential sources for water in the laminate including permeation through the acrylic surface and introduction from the spray up process. Water can cause hydrolysis of the polyester resin in the laminate, which generates new organic products. Their vapor pressure, which increases with temperature, creates the necessary pressure for blisters. Spas reinforced with polyurethane can blister since the reaction of water and methylene diphenyl diisocyanate (MDI) generates carbon dioxide, which can generate pressure sufficient for blister formation. The solution to blisters in the marine industry included the use of vinyl esters as a barrier coat behind the gel coat, and improved quality control in materials and procedures. Vinyl ester is an addition reaction polymer, and is less affected by water. Procedures to eliminate water and air in the laminate structure must also be defined and followed. These solutions were brought to the spa industry, and the frequency of blisters in portable spas has greatly declined. |
|
| The Following Illustrates (side cut of spa shell) Good not Optimum Application | |
|
|
|
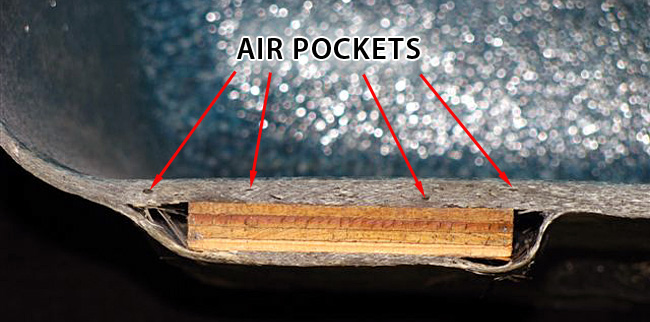
High production FRP structures laid up with chopper applications inherently have many air pockets. The barrier-skin coat process inevitably slows down the high-speed production process associated with this controlled chop lay up process at this critical stage, and will assure quality. If calculated correctly the barrier coat layers should have less fiber content than the structural layers. Thus, giving way for efficient roll-out and better saturation of the fibers. The end result is fewer airpockets in the skin and barrier layer. Follow standard manufacturing processes as well as seek out recommendations from the resin supplier(s). |
|
| The Following Illustrates (side cut of spa shell) Foot Well Support. | |
 |
|
|
Wood supports and rope embedded into the structure can make way for blisters as well, especially when the laminate is too thin allowing the material too close to the surface membrane. |
|
|
RELATED INFORMATION: |
Categories
- Bath & Shower
- Spa and Hot Tub
- Quick Glaze System
- MMA System
- Repair Filler Putty
- Surface Prep
- Texturing & Safety Additive
- Acrylic & Plastics Adhesives
- K2000 Products
- Acrylic & Gelcoat DIY Pastes
- Gelcoat Products
- Resins & Pastes
- Resin Additives
- Resin Thickeners
- Fiberglass
- Foam Support
- FRL Refinishing System
- Solvents & Strippers
- Laminating Tools & Supplies
- Plastic Jars & Bottles
- Glass Jars & Bottles
- Mixing Cups & Buckets
- Metal Cans
- Airbrushes & Touch-up
- Spraying Accessories
- Safety & Personal Protection
- Buffing & Polishing
- Sandpaper & Abrasives
- Repair Accessories & Tools
- Electric Tools
- DIY Products
- Instructional Videos
Quick Links
- Technical Info
- Applications
- About Us

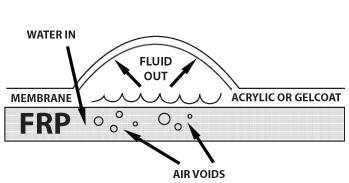 Osmosis is a very common natural phenomenon. It involves a membrane and liquids or liquid mixtures that can or cannot pass through that membrane. In the case of FRP laminates, gel coat acts as a membrane. Water permeates through the gel coat to saturate it, but also to saturate the laminate behind it. Sometimes, laminate layers within the structure work as the membrane giving way for a deeper blister. Polyester resins are produced using condensation polymerization. By using special alcohols, such as a glycol, in a reaction with di-basic acids, a polyester and water will be produced. This reaction, together with the addition of compounds such as saturated di-basic acids and cross-linking monomers, forms the basic process of polyester manufacture. As a result there is a whole range of polyesters made from different acids, glycols and monomers, all having varying properties.
Osmosis is a very common natural phenomenon. It involves a membrane and liquids or liquid mixtures that can or cannot pass through that membrane. In the case of FRP laminates, gel coat acts as a membrane. Water permeates through the gel coat to saturate it, but also to saturate the laminate behind it. Sometimes, laminate layers within the structure work as the membrane giving way for a deeper blister. Polyester resins are produced using condensation polymerization. By using special alcohols, such as a glycol, in a reaction with di-basic acids, a polyester and water will be produced. This reaction, together with the addition of compounds such as saturated di-basic acids and cross-linking monomers, forms the basic process of polyester manufacture. As a result there is a whole range of polyesters made from different acids, glycols and monomers, all having varying properties.